Electrochemical and chemical synthesis of different types of sulfonamide derivatives of N,N-dimethyl-1,4-benzenediamine using 4-nitroso-N,N-dimethylaniline†
Abstract
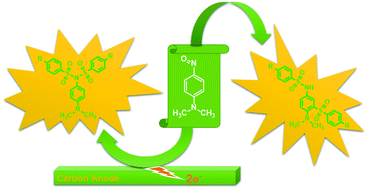
Syntheses of two different types of disulfonamide and sulfonamide derivatives of N,N-dimethyl-1,4-benzenediamine were carried out by the electrochemical oxidation of 4-nitroso-N,N-dimethylaniline in the presence of arylsulfinic acids as nucleophiles and by the chemical reaction of 4-nitroso-N,N-dimethylaniline with arylsulfinic acids. The electrochemical synthesis was carried out in aqueous ethanol at pH 7.0 and gave the pure N,N-diarylsulfonyl derivatives in 55–76% yield. The chemical synthesis, carried out in water at pH 2.0, provided the pure N-arylsulfonyl-3-arylsulfonyl derivatives in 75–85% yield.

 Please wait while we load your content...
Please wait while we load your content...