Characterization of an alkaline earth metal-doped solid superacid and its activity for the esterification of oleic acid with methanol†
Abstract
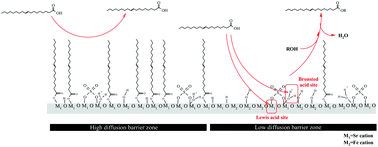
The leaching of grafted sulfate groups is one of the major issues for the solid acid catalysts with sulfate modification. A detailed study of sulfated alkaline earth metal–ferric composite oxides was carried out to suppress the leaching of sulfate groups as well as maintain high reactivity during the esterification of oleic acid. The acid properties and quantities of the active sites present on the catalysts were studied with pyridine-adsorption, FT-IR, TGA, and titration methods. The following order of acidic strength was observed: SO42−/Sr–Fe oxide > SO42−/Ca–Fe oxide > SO42−/Mg–Fe oxide. After sulfate modification, sulfate ions are linked to the composite oxides in a bridged bidentate form. SO42−/Sr–Fe oxide exhibits superacidic nature due to the high induction effect of the sulfated ion grafted on the Sr cation. TGA and FT-IR spectroscopic analyses provide new insights into the function of the iron cations implanted on the catalyst surface for enhancing the turnover frequency (TOF) of the active sites on SO42−/Sr–Fe oxide in the esterification. It was verified that the active energy of SO42−/Sr–Fe oxide for the esterification of oleic acid with methanol was as low as 28.53 ± 0.72 kJ mol−1. In the reusability study, SO42−/Sr–Fe oxide exhibited high reusability in the esterification, due to the high stability of the sulfate ion grafted on SO42−/Sr–Fe oxide.

 Please wait while we load your content...
Please wait while we load your content...