Glycerol acetals and ketals as bio-based solvents: positioning in Hansen and COSMO-RS spaces, volatility and stability towards hydrolysis and autoxidation†
Abstract
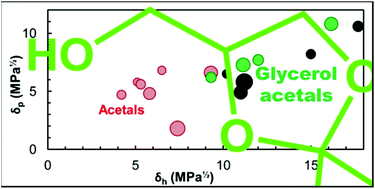
Four recently launched cyclic glycerol acetals or ketals are evaluated as bio-based solvents. Three of them are industrially available and result from the condensation of glycerol with formaldehyde, acetone and isobutyl methyl ketone. The fourth is under development and is prepared by the reaction of glycerol with benzaldehyde under heterogeneous acidic catalysis. Their solvent properties are evaluated through Hansen and COSMO-RS (COnductor-like Screening MOdel for Real Solvents) approaches, in comparison with traditional petrochemical solvents. Dioxolane- and dioxane-type isomers have close solubility parameters; however the nature of the starting aldehyde/ketone significantly impacts the solvency properties. The stability to hydrolysis depends heavily on both the aldehyde/ketone part and on the size of the ring. In acidic medium, acetals are found to be more stable than ketals and glycerol-based ketals are more stable than ethylene glycol-based ketals. In the case of benzaldehyde glycerol acetal, it is shown that the 6-membered ring isomer (dioxane-type) is approximately 8 times more stable than the 5-membered ring counterpart (dioxolane-type) at low pH. Stability towards autoxidation by O2 is high for formaldehyde and acetone-derived acetals and drops for the other two compounds. Glycerol acetals and ketals are promising potential alternatives to some harmful solvents such as glycol ethers and aniline.

 Please wait while we load your content...
Please wait while we load your content...