Electrocatalytic upgrading of model lignin monomers with earth abundant metal electrodes†
Abstract
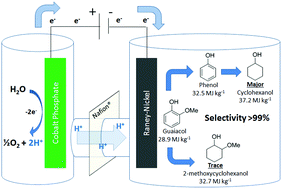
Guaiacol (2-methoxyphenol) and related lignin model monomers undergo electrocatalytic hydrogenolysis/hydrogenation (ECH) to cyclohexanol with RANEY® Nickel electrodes in aqueous solution. Aryl ether (C–O) bond cleavage is followed by reduction of the aromatic ring at ambient pressure and 75 °C. Related arene-OR cleavages occur at similar rates regardless of R-group size. Protons are supplied by anodic water oxidation on a stainless steel grid coated with cobalt-phosphate catalyst, inexpensively replacing the conventional platinum anode, and remaining viable in constant current electrolyses of up to 16 hours. The overall method addresses two key barriers to energy upgrading of low specific energy biomass into fuels and chemicals: deoxygenation and hydrogenation. By directly and simply coupling energy from renewable electricity into the chemical fuel cycle, ECH bypasses the complexity, capital costs and challenging conditions of classical H2 hydrotreating, and may help open the door to truly carbon-retentive displacement of fossil petroleum by renewables.

 Please wait while we load your content...
Please wait while we load your content...