The Paal–Knorr reaction revisited. A catalyst and solvent-free synthesis of underivatized and N-substituted pyrroles
Abstract
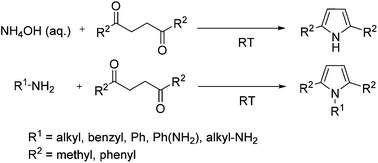
A new, modified synthesis of pyrroles is described. The reaction of 2,5-hexandione with a variety of amines yielded the expected pyrrole analogues in excellent yields. The reactions were carried out under the ultimate green conditions excluding both catalyst and solvent applying simple stirring at room temperature. The variety of amines include aqueous ammonium hydroxide for the synthesis of pyrroles with a free NH group, and benzylamines, anilines and phenylene-diamines for the synthesis of several N-derivatized pyrroles. The reaction also occurs efficiently with a variety of 1,4-diketones, although the reaction rates and yields are lower for the diketones that do not possess terminal methyl group(s).

 Please wait while we load your content...
Please wait while we load your content...