Mechanistic insights into the hydroconversion of cinnamaldehyde using mechanochemically-synthesized Pd/Al-SBA-15 catalysts
Abstract
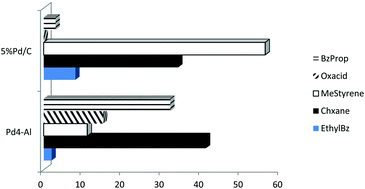
The hydroconversion of cinnamaldehyde as an α,β-unsaturated compound was studied using a simple and efficient hydrogen-donating protocol catalyzed by mechanochemically synthesized bifunctional Pd/Al-SBA-15 catalysts. Materials were characterized using TGA-TDA, nitrogen physisorption, TEM and EDX analyses. Catalytic results pointed to the presence of competitive pathways that are able to provide a selectivity switch from the expected fully hydrogenated aromatic ring (e.g. cyclohexane) and hydrogenated products (e.g. hydrocinnamaldehyde, cinnamyl alcohol and 3-phenylpropan-1-ol) to the unexpected ethylbenzene and oxalic acid products from hydrodeformylation and hydrocarboxylation reactions of cinnamaldehyde and formic acid, respectively.

 Please wait while we load your content...
Please wait while we load your content...