Activation of Nrf2 target enzymes conferring protection against oxidative stress in PC12 cells by ginger principal constituent 6-shogaol
Abstract
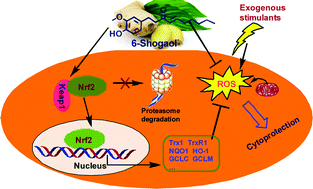
Natural compounds containing phenoxyl groups and/or Michael acceptor units appear to possess antioxidant and cytoprotective properties. The ginger principal constituent 6-shogaol (6-S) represents one of such compounds. In this study, we reported that 6-S efficiently scavenges various free radicals in vitro, and displays remarkable cytoprotection against oxidative stress-induced cell damage in the neuron-like rat pheochromocytoma cell line, PC12 cells. Pretreatment of PC12 cells with 6-S significantly upregulates a series of phase II antioxidant molecules, such as glutathione, heme oxygenase 1, NAD(P)H: quinone oxidoreductase 1, thioredoxin reductase 1, and thioredoxin 1. A mechanistic study revealed that 6-S enhanced the translocation of Nrf2 from the cytosol to the nucleus and knockdown of Nrf2 abolished such protection, indicating that this cytoprotection is mediated by the activation of the transcription factor Nrf2. Another ginger constituent 6-gingerol (6-G), having a similar structure of 6-S but lacking the alpha,beta-unsaturated ketone structure (Michael acceptor moiety), failed to shelter PC12 cells from oxidative stress. Our results demonstrate that 6-S is a novel small molecule activator of Nrf2 in PC12 cells, and suggest that 6-S might be a potential candidate for the prevention of oxidative stress-mediated neurodegenerative disorders.

 Please wait while we load your content...
Please wait while we load your content...