Inactivation kinetics and mechanisms of viral and bacterial pathogen surrogates during urine nitrification†
Abstract
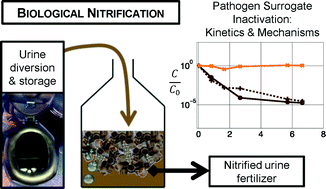
This paper assesses the inactivation performance and mechanisms in urine nitrification reactors using bacteria and bacteriophages as surrogates for human pathogens. Two parallel continuous-flow moving bed biofilm reactors (MBBRs) were operated over a two-month period. One MBBR was used to conduct a continuous spike experiment with bacteriophage MS2. The second reactor provided the matrix for a series of batch experiments conducted to investigate the inactivation of Salmonella typhimurium, Enterococcus spp., MS2, Qβ, and ΦX174 during urine nitrification. The roles of aeration, biological activity, and solution composition in inactivation were evaluated. Whereas bacteriophages ΦX174 and MS2 remained infective following urine nitrification, partial inactivation of bacteriophage Qβ was observed. Qβ inactivation was attributed primarily to aeration with a potential additive effect of biological processes, i.e., processes that are attributable to the presence of other microorganisms such as sorption to biomass, predation or enzymatic activity. Tailing of Qβ inactivation to a plateau indicated a protective effect of the solution components in aerated nitrification reactors. In contrast to the bacteriophages, S. typhimurium and Enterococcus spp. were mainly affected by biological processes: they were inactivated in biologically active nitrification reactors while remaining stable in chemically equivalent filtered controls. The tested bacteria could, for example, be out-competed by other microbial communities or sorbed to biomass in the reactor. Microbial communities did not adapt to inactivate bacteriophage MS2 (e.g., via increased prevalence of virus predators) in the experimental time-scale evaluated, with no observed inactivation of MS2 during continuous input for 51 days in the flow-through MBBR. The compilation of these results suggests that biological nitrification as a fertilizer production process remains insufficient as a stand-alone technology for the sanitization of source-separated urine.

 Please wait while we load your content...
Please wait while we load your content...