Biological versus mineralogical chromium reduction: potential for reoxidation by manganese oxide†
Abstract
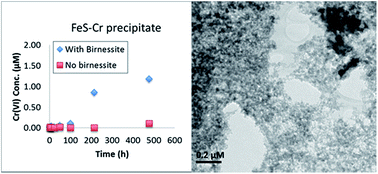
Hexavalent chromium (Cr(VI), present predominantly as CrO42− in water at neutral pH) is a common ground water pollutant, and reductive immobilization is a frequent remediation alternative. The Cr(III) that forms upon microbial or abiotic reduction often co-precipitates with naturally present or added iron (Fe), and the stability of the resulting Fe–Cr precipitate is a function of its mineral properties. In this study, Fe–Cr solids were formed by microbial Cr(VI) reduction using Desulfovibrio vulgaris strain RCH1 in the presence of the Fe-bearing minerals hematite, aluminum substituted goethite (Al-goethite), and nontronite (NAu-2, Clay Minerals Society), or by abiotic Cr(VI) reduction by dithionite reduced NAu-2 or iron sulfide (FeS). The properties of the resulting Fe–Cr solids and their behavior upon exposure to the oxidant manganese (Mn) oxide (birnessite) differed significantly. In microcosms containing strain RCH1 and hematite or Al-goethite, there was significant initial loss of Cr(VI) in a pattern consistent with adsorption, and significant Cr(VI) was found in the resulting solids. The solid formed when Cr(VI) was reduced by FeS contained a high proportion of Cr(III) and was poorly crystalline. In microcosms with strain RCH1 and hematite, Cr precipitates appeared to be concentrated in organic biofilms. Reaction between birnessite and the abiotically formed Cr(III) solids led to production of significant dissolved Cr(VI) compared to the no-birnessite controls. This pattern was not observed in the solids generated by microbial Cr(VI) reduction, possibly due to re-reduction of any Cr(VI) generated upon oxidation by birnessite by active bacteria or microbial enzymes. The results of this study suggest that Fe–Cr precipitates formed in groundwater remediation may remain stable only in the presence of active anaerobic microbial reduction. If exposed to environmentally common Mn oxides such as birnessite in the absence of microbial activity, there is the potential for rapid (re)formation of dissolved Cr(VI) above regulatory levels.

 Please wait while we load your content...
Please wait while we load your content...