A low temperature molten salt process for aluminothermic reduction of silicon oxides to crystalline Si for Li-ion batteries†
Abstract
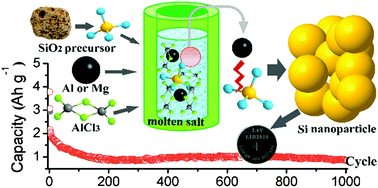
A low temperature molten salt process is developed to prepare crystalline Si nanoparticles through the reduction of micro-sized high silicon zeolite by metallic Al (or Mg) in molten AlCl3. The reaction can be initiated at 200 °C, and the yield is about 40%. As the reaction temperature increases to 250 °C, the yield can reach about 75%. When the prepared Si was used as an anode for Li-ion batteries, reversible capacities of 2663 mA h g−1 at 0.5 A g−1 after 50 cycles and 870 mA h g−1 at 3 A g−1 after 1000 cycles can be obtained. Similarly, this synthetic strategy is employed to synthesize Si nanoparticles starting from various abundant raw materials including SiO2 powder, kieselguhr, fiberglass, and even the natural mineral of albite.

 Please wait while we load your content...
Please wait while we load your content...