Solid-state activation of Li2O2 oxidation kinetics and implications for Li–O2 batteries†
Abstract
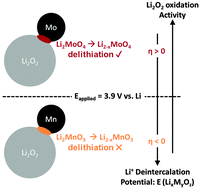
As one of the most theoretically promising next-generation chemistries, Li–O2 batteries are the subject of intense research to address their stability, cycling, and efficiency issues. The recharge kinetics of Li–O2 are especially sluggish, prompting the use of metal nanoparticles as reaction promoters. In this work, we probe the underlying pathway of kinetics enhancement by transition metal and oxide particles using a combination of electrochemistry, X-ray absorption spectroscopy, and thermochemical analysis in carbon-free and carbon-containing electrodes. We highlight the high activity of the group VI transition metals Mo and Cr, which are comparable to noble metal Ru and coincide with XAS measured changes in surface oxidation state matched to the formation of Li2MoO4 and Li2CrO4. A strong correlation between conversion enthalpies of Li2O2 with the promoter surface (Li2O2 + MaOb ± O2 → LixMyOz) and electrochemical activity is found that unifies the behaviour of solid-state promoters. In the absence of soluble species on charge and the decomposition of Li2O2 proceeding through solid solution, enhancement of Li2O2 oxidation is mediated by chemical conversion of Li2O2 with slow oxidation kinetics to a lithium metal oxide. Our mechanistic findings provide new insights into the selection and/or employment of electrode chemistry in Li–O2 batteries.


 Please wait while we load your content...
Please wait while we load your content...