Pyrite FeS2 for high-rate and long-life rechargeable sodium batteries†
Abstract
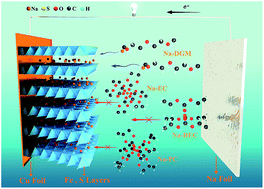
It is desirable to develop electrode materials for advanced rechargeable batteries with low cost, long life, and high-rate capability. Pyrite FeS2, as an easily obtained natural mineral, has been already commercialized in primary lithium batteries, but encountered problems in rechargeable batteries with carbonate-based electrolytes due to the limited cycle life caused by the conversion-type reaction (FeS2 + 4M → Fe + 2M2S (M = Li or Na)). Herein, we demonstrate that FeS2 microspheres can be applied in room-temperature rechargeable sodium batteries with only the intercalation reaction by simultaneously selecting a compatible NaSO3CF3/diglyme electrolyte and tuning the cut-off voltage to 0.8 V. A surprisingly high-rate capability (170 mA h g−1 at 20 A g−1) and unprecedented long-term cyclability (∼90% capacity retention for 20 000 cycles) has been obtained. We suggest that a stable electrically conductive layer-structured NaxFeS2 was formed during cycling, which enables the highly reversible sodium intercalation and deintercalation. Moreover, 18650-type sodium batteries were constructed exhibiting a high capacity of ∼4200 mA h (corresponding to 126 W h kg−1 and 382 W h L−1) and a capacity retention of 97% after an initial 200 cycles at 4 A during charge–discharge. This shows that the production of rechargeable sodium batteries with FeS2 microspheres is viable for commercial utilization.

 Please wait while we load your content...
Please wait while we load your content...