Magnetic superexchange interactions: trinuclear bis(oxamidato) versus bis(oxamato) type complexes†
Abstract
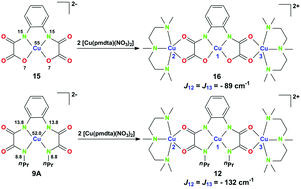
The diethyl ester of o-phenylenebis(oxamic acid) (opbaH2Et2) was treated with an excess of RNH2 in MeOH to cause the exclusive formation of the respective o-phenylenebis(N(R)-oxamides) (opboH4R2, R = Me 1, Et 2, nPr 3) in good yields. Treatment of 1–3 with half an equivalent of [Cu2(AcO)4(H2O)2] or one equivalent of [Ni(AcO)2(H2O)4] followed by the addition of four equivalents of [nBu4N]OH resulted in the formation of mononuclear bis(oxamidato) type complexes [nBu4N]2[M(opboR2)] (M = Ni, R = Me 4, Et 5, nPr 6; M = Cu, R = Me 7, Et 8, nPr 9). By addition of two equivalents of [Cu(pmdta)(NO3)2] to MeCN solutions of 7–9, novel trinuclear complexes [Cu3(opboR2)(L)2](NO3)2 (L = pmdta, R = Me 10, Et 11, nPr 12) could be obtained. Compounds 4–12 have been characterized by elemental analysis and NMR/IR spectroscopy. Furthermore, the solid state structures of 4–10 and 12 have been determined by single-crystal X-ray diffraction studies. By controlled cocrystallization, diamagnetically diluted 8 and 9 (1%) in the host lattice of 5 and 6 (99%) (8@5 and 9@6), respectively, in the form of single crystals have been made available, allowing single crystal ESR studies to extract all components of the g-factor and the tensors of onsite CuA and transferred NA hyperfine (HF) interaction. From these studies, the spin density distribution of the [Cu(opboEt2)]2− and [Cu(opbonPr2)]2− complex fragments of 8 and 9, respectively, could be determined. Additionally, as a single crystal ENDOR measurement of 8@5 revealed the individual HF tensors of the N donor atoms to be unequal, individual estimates of the spin densities on each N donor atom were made. The magnetic properties of 10–12 were studied by susceptibility measurements versus temperature to give J values varying from −96 cm−1 (10) over −104 cm−1 (11) to −132 cm−1 (12). These three trinuclear CuII-containing bis(oxamidato) type complexes exhibit J values which are comparable to and slightly larger in magnitude than those of related bis(oxamato) type complexes. In a summarizing discussion involving experimentally obtained ESR results (spin density distribution) of 8 and 9, the geometries of the terminal [Cu(pmdta)]2+ fragments of 12 determined by crystallographic studies, together with accompanying quantum chemical calculations, an approach is derived to explain these phenomena and to conclude if the spin density distribution of mononuclear bis(oxamato)/bis(oxamidato) type complexes could be a measure of the J couplings of corresponding trinuclear complexes.

 Please wait while we load your content...
Please wait while we load your content...