1,2,4-Triazole-derived carbene complexes of gold: characterization, solid-state aggregation and ligand disproportionation†
Abstract
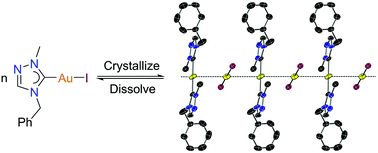
Ligand redistribution reactions are well documented for silver(I) N-heterocyclic carbene (NHC) complexes of the type [AgX(NHC)] (X = halido ligand), but only two reports have been described in the literature for gold analogues of the general formula [AuX(NHC)]. In both cases, the NHCs in question were exceptionally strong donors. To probe the dependence of ligand redistribution processes on NHC donor strength, a model study was conducted using a weakly donating 1,2,4-triazolin-5-ylidene (tazy) ligand and different halido coligands. For [AuX(tazy)] (X = Cl, Br, OAc, tazy = 4-benzyl-1-methyl-1,2,4-triazolin-5-ylidene), no ligand redistribution was found, while a reversible disproportionation between [AuI(tazy)] in solution and [Au(tazy)2][AuI2] in the solid state was observed and studied by means of X-ray crystallography, NMR and UV-Vis spectroscopy, as well as DFT calculations.
- This article is part of the themed collection: New Talent: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...