Exploring the reactivity of manganese(iii) complexes with diphenolate-diamino ligands in rac-lactide polymerization†
Abstract
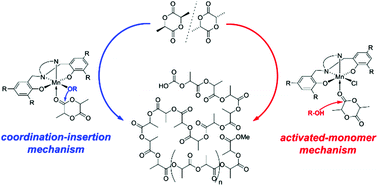
Manganese(III) complexes of tetradentate diphenolate-diamino (NNOO2−) ligands were prepared from aerobic reaction of MnCl2 with the respective ligands in basic methanolic solution. Methoxide complexes (NNOO)Mn(OMe)(MeOH)0–1 were obtained for three ligands, while others only provided the respective chloride complexes (NNOO)Mn(Cl)(MeOH). Complexes were analyzed by X-ray diffraction studies and octahedral complexes showed evidence of Jahn–Teller distortions. Magnetic moments determined in MeOD were indicative of high-spin Mn(III)-d4 complexes (μeff = 4.2–4.6μB). Methoxide complexes were active in the coordination–insertion polymerization of rac-lactide (130 °C, 0.33–1.0 mol% catalyst loading) to yield atactic polylactic acid with moderate molecular weight control. Polymerization activity was reduced, but not suppressed by the presence of protic impurities. Chloride complexes showed less activity and only in the presence of external alcohol, indicative of an activated-monomer mechanism.

 Please wait while we load your content...
Please wait while we load your content...