Group 1 and group 2 metal complexes supported by a bidentate bulky iminopyrrolyl ligand: synthesis, structural diversity, and ε-caprolactone polymerization study†
Abstract
We report here a series of alkali and alkaline earth metal complexes, each with a bulky iminopyrrolyl ligand [2-(Ph3CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3NH] (1-H) moiety in their coordination sphere, synthesized using either alkane elimination or silylamine elimination methods or the salt metathesis route. The lithium salt of molecular composition [Li(2-(Ph3CN
CH)C4H3NH] (1-H) moiety in their coordination sphere, synthesized using either alkane elimination or silylamine elimination methods or the salt metathesis route. The lithium salt of molecular composition [Li(2-(Ph3CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N)(THF)2] (2) was prepared using the alkane elimination method, and the silylamine elimination method was used to synthesize the dimeric sodium and tetra-nuclear potassium salts of composition [(2-(Ph3CN
CH)C4H3N)(THF)2] (2) was prepared using the alkane elimination method, and the silylamine elimination method was used to synthesize the dimeric sodium and tetra-nuclear potassium salts of composition [(2-(Ph3CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N)Na(THF)]2 (3) and [(2-(Ph3CN
CH)C4H3N)Na(THF)]2 (3) and [(2-(Ph3CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N)K(THF)0.5]4 (4) respectively. The magnesium complex of composition [(THF)2Mg(CH2Ph){2-(Ph3CN
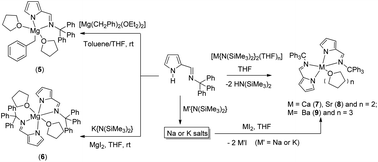
CH)C4H3N)K(THF)0.5]4 (4) respectively. The magnesium complex of composition [(THF)2Mg(CH2Ph){2-(Ph3CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N}] (5) was synthesized through the alkane elimination method, in which [Mg(CH2Ph)2(OEt2)2] was treated with the bulky iminopyrrole ligand 1-H in 1 : 1 molar ratio, whereas the bis(iminopyrrolyl)magnesium complex [(THF)2Mg{2-(Ph3CN
CH)C4H3N}] (5) was synthesized through the alkane elimination method, in which [Mg(CH2Ph)2(OEt2)2] was treated with the bulky iminopyrrole ligand 1-H in 1 : 1 molar ratio, whereas the bis(iminopyrrolyl)magnesium complex [(THF)2Mg{2-(Ph3CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N}2] (6) was isolated using the salt metathesis route. The heavier alkaline earth metal complexes of the general formula {(THF)nM(2-(Ph3CN
CH)C4H3N}2] (6) was isolated using the salt metathesis route. The heavier alkaline earth metal complexes of the general formula {(THF)nM(2-(Ph3CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C4H3N)2} [M = Ca (7), Sr (8), and n = 2; M = Ba (9), n = 3] were prepared in pure form using two synthetic methods: in the first method, the bulky iminopyrrole ligand 1-H was directly treated with the alkaline earth metal precursor [M{N(SiMe3)2}2(THF)n] (where M = Ca, Sr and Ba) in 2 : 1 molar ratio in THF solvent at ambient temperature. The complexes 7–9 were also obtained using the salt metathesis reaction, which involves the treatment of the potassium salt (4) with the corresponding metal diiodides MI2 (M = Ca, Sr and Ba) in 2 : 1 molar ratio in THF solvent. The molecular structures of all the metal complexes (1-H, 2–9) in the solid state were established through single-crystal X-ray diffraction analysis. The complexes 5–9 were tested as catalysts for the ring-opening polymerization of ε-caprolactone. High activity was observed in the heavier alkaline earth metal complexes 7–9, with a very narrow polydispersity index in comparison to that of magnesium complexes 5 and 6.
CH)C4H3N)2} [M = Ca (7), Sr (8), and n = 2; M = Ba (9), n = 3] were prepared in pure form using two synthetic methods: in the first method, the bulky iminopyrrole ligand 1-H was directly treated with the alkaline earth metal precursor [M{N(SiMe3)2}2(THF)n] (where M = Ca, Sr and Ba) in 2 : 1 molar ratio in THF solvent at ambient temperature. The complexes 7–9 were also obtained using the salt metathesis reaction, which involves the treatment of the potassium salt (4) with the corresponding metal diiodides MI2 (M = Ca, Sr and Ba) in 2 : 1 molar ratio in THF solvent. The molecular structures of all the metal complexes (1-H, 2–9) in the solid state were established through single-crystal X-ray diffraction analysis. The complexes 5–9 were tested as catalysts for the ring-opening polymerization of ε-caprolactone. High activity was observed in the heavier alkaline earth metal complexes 7–9, with a very narrow polydispersity index in comparison to that of magnesium complexes 5 and 6.


 Please wait while we load your content...
Please wait while we load your content...