Coordination chemistry of 2,2′-biphenylenedithiophosphinate and diphenyldithiophosphinate with U, Np, and Pu†
Abstract
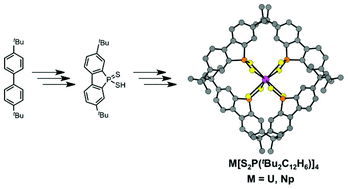
New members of the dithiophosphinic acid family of potential actinide extractants were prepared: heterocyclic 2,2′-biphenylenedithiophosphinic acids of stoichiometry HS2P(R2C12H6) (R = H or tBu). The time- and atom-efficient syntheses afforded multigram quantities of pure HS2P(R2C12H6) in reasonable yields (∼60%). These compounds differed from other diaryldithiophosphinic acid extractants in that the two aryl groups were connected to one another at the ortho positions to form a 5-membered dibenzophosphole ring. These 2,2′-biphenylenedithiophosphinic acids were readily deprotonated to form S2P(R2C12H6)1− anions, which were crystallized as salts with tetraphenylpnictonium cations (ZPh41+; Z = P or As). Coordination chemistry between [S2P(tBu2C12H6)]1− and [S2P(C6H5)2]1− with U, Np, and Pu was comparatively investigated. The results showed that dithiophosphinate complexes of UIV and NpIV were redox stable relative to those of UIII, whereas reactions involving PuIV gave intractable material. For instance, reactions involving UIV and NpIV generated An[S2P(tBu2C12H6)]4 and An[S2P(C6H5)2]4 whereas reactions between PuIV and [S2P(C6H5)2]1− generated a mixture of products from which we postulated a transient PuIII species based on UV-Vis spectroscopy. However, the trivalent Pu[S2P(C6H5)2]3(NC5H5)2 compound is stable and could be isolated from reactions between [S2P(C6H5)2]1− and the trivalent PuI3(NC5H5)4 starting material. Attempts to synthesize analogous trivalent compounds with UIII provided the tetravalent U[S2P(C6H5)2]4 oxidation product.

 Please wait while we load your content...
Please wait while we load your content...