Nitronyl nitroxide based 2p–3d–4f chains with the magnetocaloric effect and slow magnetic relaxation†
Abstract
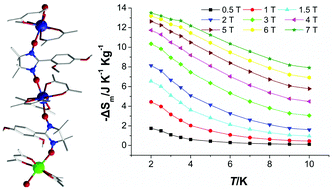
Four new nitronyl nitroxide radical based hetero-tri-spin one-dimensional compounds, namely [{Ln(hfac)3}3{Cu(hfac)2}{NIT–Ph(OMe)2}4]n (Ln = Gd (1), Tb (2), Dy (3), Er (4); hfac = hexafluoroacetylacetonate; NIT–Ph(OMe)2 = 2-(2′,4′-dimethoxyphenyl)-4,4,5,5-tetramethyl-imidazolyl-1-oxyl-3-oxide) have been successfully prepared. Single crystal X-ray crystallographic analysis reveals that complexes 1–4 possess a 1D chain structure with a repeating [Cu–Rad–Ln–Rad–Ln–Rad–Ln–Rad] moiety in which Ln(hfac)3 and Cu(hfac)2 units are bridged by nitronyl nitroxide radicals through the NO groups. DC magnetic studies found that ferromagnetic interactions between metals and the coordinated NO groups are active in all four compounds. The Tb derivative displays frequency dependent ac magnetic susceptibilities, indicating slow magnetic relaxation behavior. The Gd complex shows an important cryogenic magnetocaloric effect with the entropy change (−ΔSm) of 13.5 J kg−1 K−1 at 2 K and a magnetic field of 7 T, representing the first example of Gd–radical molecular species exhibiting the magnetocaloric effect.

 Please wait while we load your content...
Please wait while we load your content...