Structural diversity of late transition metal complexes with flexible tetra-NHC ligands†
Abstract
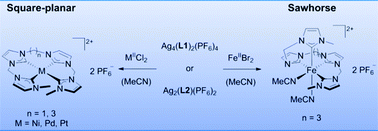
The synthesis of copper, gold, nickel, palladium, platinum, and iron complexes with open chain tetra-N-heterocyclic carbene (NHC) ligands via transmetalation using silver NHC complexes is presented. The obtained complexes show differing coordination geometries depending on both ligand structure and metal. While the complexes of the coinage metals form di- or tetranuclear structures, the group 10 metal complexes exhibit a distorted square planar coordination geometry at the metal centers. In the case of iron an enhanced flexibility of the ligand – caused by a longer alkyl bridge – leads to octahedral complexes with a sawhorse-type coordination by the tetracarbene ligand and two cis acetonitrile ligands. To the best of our knowledge, this is the first known example of a tetracarbene ligand in sawhorse-type coordination within an octahedral coordination sphere. The remaining cis-labile sites are prone to exchange reactions as shown by addition of trimethylphosphine.

 Please wait while we load your content...
Please wait while we load your content...