Facile synthesis of anhydrous alkaline earth metal dodecaborates MB12H12 (M = Mg, Ca) from M(BH4)2†
Abstract
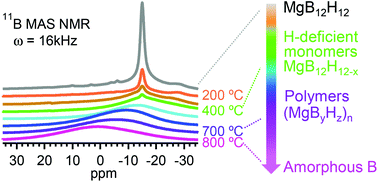
Metal dodecaborates M2/nB12H12 are among the dehydrogenation intermediates of metal borohydrides M(BH4)n with a high hydrogen density of approximately 10 mass%, the latter is a potential hydrogen storage material. There is therefore a great need to synthesize anhydrous M2/nB12H12 in order to investigate the thermal decomposition of M2/nB12H12 and to understand its role in the dehydrogenation and rehydrogenation of M(BH4)n. In this work, anhydrous alkaline earth metal dodecaborates MB12H12 (M = Mg, Ca) have been successfully synthesized by sintering M(BH4)2 (M = Mg, Ca) and B10H14 in a stoichiometric molar ratio of 1 : 1. Thermal decomposition of MB12H12 shows multistep pathways with the formation of H-deficient monomers MB12H12−x containing icosahedral B12 skeletons and is followed by the formation of (MByHz)n polymers. Comparison of the thermal decomposition of MB12H12 and M(BH4)2 suggests different behaviours of the anhydrous MB12H12 and those formed from the decomposition of M(BH4)n.

 Please wait while we load your content...
Please wait while we load your content...