Self-assembly of oxamidato bridged ester functionalised dirhenium metallastirrups: synthesis, characterisation and cytotoxicity studies†‡
Abstract
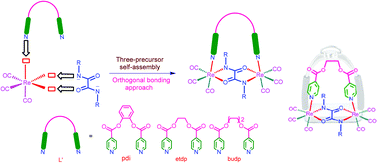
A new set of ester functionalised Re(I)-based oxamidato bridged neutral dinuclear metallacycles were synthesised by self-assembly of four components from three building blocks in a facile one-pot reaction via an orthogonal bonding approach. Oxidative addition of oxamide ligands (H2L = N,N′-diphenyloxamide, and N,N′-dibenzyloxamide) to rhenium carbonyl (Re2(CO)10) in the presence of semi-rigid and flexible ditopic pyridyl ligands (L′ = o-phenylene diisonicotinate (pdi), ethane diyl di-4-pyridine carboxylate (etdp) and 1,4-butane diyl di-4-pyridine carboxylate (budp)) having ester functionality afforded neutral dirhenium metallacycles of the general formula [(CO)3Re(μ-L)(μ-L′)Re(CO)3] (1–5) under solvothermal reaction conditions. The metallacyclic compounds were characterised using elemental analyses, IR, UV–vis and NMR spectroscopic techniques. Structural analyses of 2–5 by single crystal X-ray diffraction methods revealed a stirrup like molecular framework in which two fac-Re(CO)3 units are bridged together by dissymmetrical NO∩ON bis-chelation of oxamide ligands (as a pedestal of stirrups) and further connected by a flexible ditopic tecton (as an arched anchor of stirrups) in an orthogonal fashion. The cytotoxicity activities of dirhenium metallacycles 1–5 were studied in vitro against three different cancer cell lines and normal cells.


 Please wait while we load your content...
Please wait while we load your content...