Highly luminescent charge-neutral europium(iii) and terbium(iii) complexes with tridentate nitrogen ligands†
Abstract
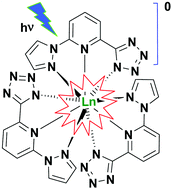
We report on the synthesis of tridentate-nitrogen pyrazole–pyridine–tetrazole (L1H) and pyrazole–pyridine–triazole (L2H) ligands and their complexation with lanthanides (Ln = Gd(III), Eu(III) and Tb(III)) resulting in stable, charge-neutral complexes Ln(L1)3 and Ln(L2)3, respectively. X-ray crystallographic analysis of the complexes with L1 ligands revealed tricapped trigonal coordination geometry around the lanthanide ions. All complexes show bright photoluminescence (PL) in the solid state, indicating efficient sensitization of the lanthanide emission via the triplet states of the ligands. In particular, the terbium complexes show high PL quantum yields of 65 and 59% for L1 and L2, respectively. Lower PL efficiencies of the europium complexes (7.5 and 9%, respectively) are attributed to large energy gaps between the triplet states of the ligands and accepting levels of Eu(III). The triplet state energy can be reduced by introducing an electron withdrawing (EW) group at the 4 position of the pyridine ring. Such substitution of L1H with a carboxylic ester (COOMe) EW group leads to a europium complex with increased PL quantum yield of 31%. A comparatively efficient PL of the complexes dissolved in ethanol indicates that the lanthanide ions are shielded against nonradiative deactivation via solvent molecules.

 Please wait while we load your content...
Please wait while we load your content...