C–F sp2 bond functionalization mediated by niobium complexes†
Abstract
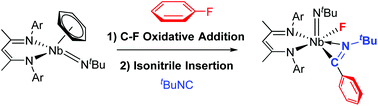
Insertion chemistry of isocyanide molecules was used to functionalize C–F sp2 bonds after their oxidative addition across the metal center in a β-diketiminate niobium(III) imido complex (BDI)Nb(NtBu)(C6H6). The complexes formed, 3a–b ([BDI]Nb(PhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)(NtBu)(F) (R = 1,6-diisopropylphenyl, tert-butyl), were characterized by NMR spectroscopy and X-ray analysis. Further treatment with phenylsilane induced H/F exchange under mild conditions, which was followed by hydride transfer to the inserted isocyanide. Divergent reactivity was observed when the two analogous aryl and tert-butyl isocyanide insertion products were treated with phenylsilane.
N)(NtBu)(F) (R = 1,6-diisopropylphenyl, tert-butyl), were characterized by NMR spectroscopy and X-ray analysis. Further treatment with phenylsilane induced H/F exchange under mild conditions, which was followed by hydride transfer to the inserted isocyanide. Divergent reactivity was observed when the two analogous aryl and tert-butyl isocyanide insertion products were treated with phenylsilane.
- This article is part of the themed collection: Fluorine

 Please wait while we load your content...
Please wait while we load your content...