Supramolecular fluorescence enhancement via coordination-driven self-assembly in bis-picolylcalixarene blue-emitting M2L2Xn macrocycles†
Abstract
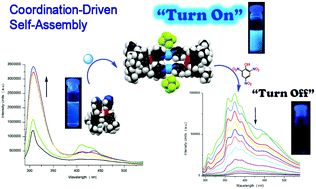
The lower-rim functionalised distal bis-(4-picolyl)-p-tert-butylcalix[4]arene (L) selectively and quantitatively self-assembles into a series of discrete [2 + 2] blue-emitting metallacycles of general formula M2L2Xn with diverse metal salts (M = Zn2+, Pd2+, Ag+, and Cd2+; X = Cl−, NO3−, ClO4−, BF4− , CF3SO3−, PF6−, SbF6−; n = 2, 4). Macrocycle assembly has been corroborated by 2D-DOSY NMR and ESI-MS analyses, which further indicate that the M2L2Xn entities are quite stable and persist as robust and discrete macrocyclic species in solution. While free L units display modest blue emission (λmax = 307–405 nm), self-assembly of M2L2Xn results in amplified fluorescence (up to 13-fold). This remarkable enhancement may be primarily ascribed to the increase in conformational rigidity imposed on the L units by supramolecular assembly formation upon metal coordination to the pyridyl groups; in addition, subtle intensity-emission modulation may be provided by the different metal components. Titrations aimed at exploring the possibilities for ratiometric detection of metal cations or sensing of nitroaromatic species, revealed that the M2L2Xn platform may be a suitable “turn-on/off” system. Our results provide valuable insights into luminescence enhancement within the context of coordination-driven assemblies, which may be engineered to increase their fluorescence by imposing rigidity on the chromophores.

 Please wait while we load your content...
Please wait while we load your content...