Bridged bis-BODIPYs: their synthesis, structures and properties†
Abstract
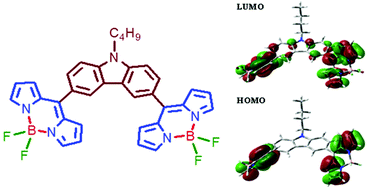
A series of bis-BODIPYs 1–6 bridged via thiophene, furan, N-alkylcarbazole, triphenyl-amine, para- and meta-phenylene groups have been synthesized and characterized by various spectroscopic techniques. The change in the spectroscopic properties of bis-BODIPYs upon varying the size of spacers was studied. X-ray crystal structures of three bis-BODIPYs containing triphenylamine, para- and meta-phenylene bridges were solved. Intermolecular C(H)⋯π and π⋯π stacking interactions were observed in solid state structures of three bis-BODIPYs. The dihedral angles between the spacer unit and two boron-dipyrrin units were lower in all three compounds as compared to their corresponding monomers. This suggests increased interactions between the two boron-dipyrrin units in molecules which are in turn reflected in the anodic shifts in their reduction potentials. DFT studies indicated effective electronic interactions between spacers and two boron dipyrrin units in all the bis-BODIPYs. The calculated HOMO–LUMO gap was found to be lower for bis-BODIPY having bulky carbazole spacers and higher for bis-BODIPY having smaller furan spacers. Changing the spacer size clearly affected the spectroscopic properties of the bis-BODIPYs and red shifted absorption and emission maxima were observed for bis-BODIPYs with furan and thiophene spacers as compared to bis-BODIPYs with phenylene or bulky aromatic spacers.

 Please wait while we load your content...
Please wait while we load your content...