Alkali–metal ion coordination in uranyl(vi) poly-peroxo complexes in solution, inorganic analogues to crown-ethers. Part 2. Complex formation in the tetramethyl ammonium-, Li+-, Na+- and K+-uranyl(vi)–peroxide–carbonate systems†
Abstract
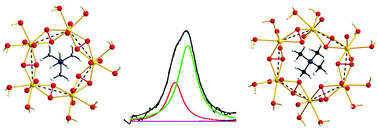
The constitution and equilibrium constants of ternary uranyl(VI) peroxide carbonate complexes [(UO2)p(O2)q(CO3)r]2(p−q−r) have been determined at 0 °C in 0.50 M MNO3, M = Li, K, and TMA (tetramethyl ammonium), ionic media using potentiometric and spectrophotometric data; 17O NMR data were used to determine the number of complexes present. The formation of cyclic oligomers, “[(UO2)(O2)(CO3)]n”, n = 4, 5, 6, with different stoichiometries depending on the ionic medium used, suggests that Li+, Na+, K+ and TMA ions act as templates for the formation of uranyl peroxide rings where the uranyl-units are linked by μ–η2–η2 bridged peroxide-ions. The templating effect is due to the coordination of the M+-ions to the uranyl oxygen atoms, where the coordination of Li+ results in the formation of Li[(UO2)(O2)(CO3)]47−, Na+ and K+ in the formation of Na/K[(UO2)(O2)(CO3)]59− complexes, while the large tetramethyl ammonium ion promotes the formation of two oligomers, TMA[(UO2)(O2)(CO3)]59− and TMA[(UO2)(O2)(CO3)]611−. The NMR spectra demonstrate that the coordination of Na+ in the five- and six-membered oligomers is significantly stronger than that of TMA+; these observations suggest that the templating effect is similar to the one observed in the synthesis of crown-ethers. The NMR experiments also demonstrate that the exchange between TMA[(UO2)(O2)(CO3)]59− and TMA[(UO2)(O2)(CO3)]611− is slow on the 17O chemical shift time-scale, while the exchange between TMA[(UO2)(O2)(CO3)]611− and Na[(UO2)(O2)(CO3)]611− is fast. There was no indication of the presence of large clusters of the type identified by Burns and Nyman (M. Nyman and P. C. Burns, Chem. Soc. Rev., 2012, 41, 7314–7367) and possible reasons for this and the implications for the synthesis of large clusters are briefly discussed.

 Please wait while we load your content...
Please wait while we load your content...