Sequential electrophilic P–C bond formation in metal-coordinated chlorophosphines†
Abstract
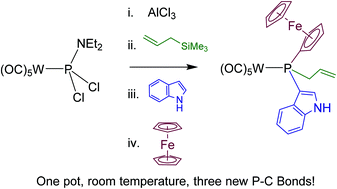
In the presence of chloride abstractors, metal-coordinated chlorophosphines undergo facile room-temperature electrophilic substitution reactions with unsaturated organic substrates, leading to P–C bond formation. This methodology can be applied sequentially two or three times, stepwise or in one-pot reactions, to form phosphines with three different substituents. The reactions are rapid and high-yielding, and can be applied to a wide range of organic substrates, making them valuable tools for P–C bond formation.

 Please wait while we load your content...
Please wait while we load your content...