Tuning of exchange coupling by the Mn–O distance and phenoxido bridging angle: an experimental and theoretical study of the family of Mn(iii) dimers with salen type ligands†
Abstract
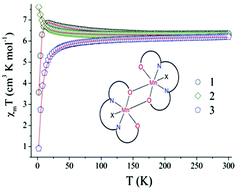
Three new Mn(III) complexes [Mn2L2(ClO4)2] (1), [Mn2L2(NCS)2] (2) and [Mn2L2N(CN)2]ClO4·CH3CN (3) have been synthesized from the Schiff base ligand H2L (where H2L = N,N′-bis(2-hydroxypropiophenone)-1,2- ethanediamine) and various anionic coligands e.g. perchlorate, thiocyanate and dicyanamide. X-ray crystal structure analysis reveals that 1 and 2 are dinuclear complexes, joined together by Mn⋯O (phenoxido) interactions. Whereas 3 consists of an alternating phenoxido and μ1,5 dicyanamido bridge, resulting in a 1D chain. In 1 and 2 ferromagnetic coupling exists between the Mn(III) centres within the dimer but 3 possesses antiferromagnetic interaction. This difference in magnetic exchange interactions has been rationalized on the basis of structural parameters like longer bridging Mn–O distance and Mn–O–Mn angle in these complexes with the help of DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...