MoCl5 as an effective chlorinating agent towards α-amino acids: synthesis of α-ammonium-acylchloride salts and α-amino-acylchloride complexes†
Abstract
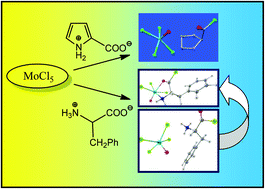
The reactivity of MoCl5 with α-amino acids was investigated for the first time by choosing CH2Cl2 as the reaction medium. The interaction of MoCl5 with L-proline proceeded with Cl/O interchange and led to the formation of [NH2(CH2)3CHC(O)Cl][MoOCl4], 1, in 63% yield. The reactions of MoCl5 with L-phenylalanine, sarcosine, N,N-dimethylglycine and N,N-dimethyl-L-phenylalanine afforded the α-amino-acylchloride complexes MoOCl3[O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Cl)CH(R)N(R′)(R′′)] (R = CH2Ph, R′ = R′′ = H, 2a; R = R′ = H, R′′ = Me, 2b; R = H, R′ = R′′ = Me, 2c; R = CH2Ph, R′ = R′′ = Me, 2d), in ca. 70% yields. According to DFT studies, 2a,d are mononuclear Mo(V) octahedral complexes bearing a N,O-coordinated α-amino-acylchloride ligand. The presumed species formed during the first stages of the MoCl5/L-phenylalanine interaction were DFT-elucidated, thus the calculated Gibbs free energy profile for the multi-step reaction of MoCl5 with L-phenylalanine was traced. [NH3CH(CH2Ph)C(O)Cl][MoOCl4], 3, acting as an intermediate in the course of the formation of 2a, was isolated by combination of [NH3CH(CH2Ph)C(O)Cl][Cl] with MoOCl3.
C(Cl)CH(R)N(R′)(R′′)] (R = CH2Ph, R′ = R′′ = H, 2a; R = R′ = H, R′′ = Me, 2b; R = H, R′ = R′′ = Me, 2c; R = CH2Ph, R′ = R′′ = Me, 2d), in ca. 70% yields. According to DFT studies, 2a,d are mononuclear Mo(V) octahedral complexes bearing a N,O-coordinated α-amino-acylchloride ligand. The presumed species formed during the first stages of the MoCl5/L-phenylalanine interaction were DFT-elucidated, thus the calculated Gibbs free energy profile for the multi-step reaction of MoCl5 with L-phenylalanine was traced. [NH3CH(CH2Ph)C(O)Cl][MoOCl4], 3, acting as an intermediate in the course of the formation of 2a, was isolated by combination of [NH3CH(CH2Ph)C(O)Cl][Cl] with MoOCl3.

 Please wait while we load your content...
Please wait while we load your content...