ZnII and HgII binding to a designed peptide that accommodates different coordination geometries†
Abstract
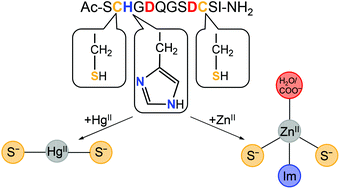
Designed metal ion binding peptides offer a variety of applications in both basic science as model systems of more complex metalloproteins, and in biotechnology, e.g. in bioremediation of toxic metal ions, biomining or as artificial enzymes. In this work a peptide (HS: Ac-SCHGDQGSDCSI-NH2) has been specifically designed for binding of both ZnII and HgII, i.e. metal ions with different preferences in terms of coordination number, coordination geometry, and to some extent ligand composition. It is demonstrated that HS accommodates both metal ions, and the first coordination sphere, metal ion exchange between peptides, and speciation are characterized as a function of pH using UV-absorption-, synchrotron radiation CD-, 1H-NMR-, and PAC-spectroscopy as well as potentiometry. HgII binds to the peptide with very high affinity in a {HgS2} coordination geometry, bringing together the two cysteinates close to each end of the peptide in a loop structure. Despite the high affinity, HgII is kinetically labile, exchanging between peptides on the subsecond timescale, as indicated by line broadening in 1H-NMR. The ZnII-HS system displays more complex speciation, involving monomeric species with coordinating cysteinates, histidine, and a solvent water molecule, as well as HS-ZnII-HS complexes. In summary, the HS peptide displays conformational flexibility, contains many typical metal ion binding groups, and is able to accommodate metal ions with different structural and ligand preferences with high affinity. As such, the HS peptide may be a scaffold offering binding of a variety of metal ions, and potentially serve for metal ion sequestration in biotechnological applications.

 Please wait while we load your content...
Please wait while we load your content...