Three bonding modes of bis(2-picolyl)phenylphosphine at iron: isolation of a dinuclear iron complex featuring dearomatized pyridine moieties†
Abstract
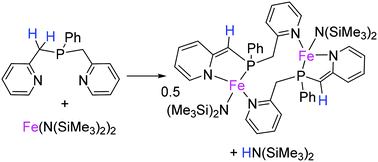
The coordination ability of the bis(2-picolyl)phenylphosphine (NPN) compound has been probed toward the iron precursor [Fe{N(SiMe3)2}2]. By a careful control of the experimental conditions we were able to isolate and characterize three complexes displaying different coordination modes of the NPN ligand as revealed in particular by X-ray diffraction, and multinuclear solution and solid state NMR analyses. It is shown that NPN can be used as a proton-responsive ligand with enough flexibility to allow the formation of the dinuclear complex [Fe{P(CH2(C5H4N))(CH(C5H4N))}(N(SiMe3)2)]2 with two NPN ligands spanning the two metal centres.

 Please wait while we load your content...
Please wait while we load your content...