Cyclic tetramers of a five-membered palladacycle based on a head-to-tail-linked isocyanate dimer and their reactivity in cyclotrimerization of isocyanates†
Abstract
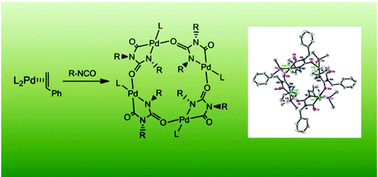
Reactions of [Pd(styrene)(PR3)2], generated from trans-[PdEt2(PR3)2] and styrene, with 2 equiv. of benzyl isocyanate in THF at room-temperature afforded unusual cyclic Pd-tetramers of five-membered rings consisting of organic isocyanate dimers and palladium, [Pd(PR3){–C(O)N(R)C(O)N(R)–}]4 (PR3 = PMe3, 1; PR3 = PMe2Ph, 2). Additionally, a cyclic trimer, (RNCO)3, 3 (R = benzyl) was produced as a catalytic product. Treatment of the cyclic tetramer (1) with 4 equiv. of chelated phosphine, such as (1,2-bis(diethylphosphino)ethane) (DEPE) or (1,2-bis(dimethylphosphino)ethane) (DMPE), readily caused conversion to a metallacyclic cis-form, [Pd{N(R)C(O)N(R)C(O)}(P ∼ P)] (P ∼ P = DEPE, 4; P ∼ P = DMPE, 5) in quantitative yields. In contrast, reactions of Pd(0)-PR3 with 2 equiv. of Ar-NCO (Ar = Ph, p-tolyl, p-ClC6H4) afforded metallacyclic complexes having a dimeric isocyanato moiety, cis-[Pd{C(O)N(Ar)–C(O)N(Ar)}(PR3)2] (PR3 = PMe3 Ar = C6H5, 6; p-MeC6H4, 7; p-Cl-C6H4, 8; PR3 = PMe2Ph, Ar = p-Cl-C6H4, 9). Treatment of the palladacyclic complex (8) with an equimolar amount of chelated phosphine such as DEPE readily caused conversion to a palladacyclic cis-form, [Pd{N(Ar)C(O)N(Ar)C(O)}(DEPE)], 10 in quantitative yield. The catalytic cyclotrimerization of benzyl isocyanate to [Pd(styrene)(PMe3)2] was achieved by varying the molar ratio of R-NCO (R = benzyl). In addition, catalytic cyclotrimerization was performed from the five-membered palladacyclic complexes or the Pd(0)-PR3 complex with excess Ar-NCO.

 Please wait while we load your content...
Please wait while we load your content...