The advantages of covalently attaching organometallic catalysts to a carbon black support: recyclable Rh(i) complexes that deliver enhanced conversion and product selectivity†
Abstract
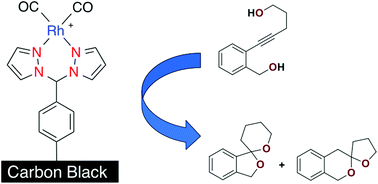
Pure carbon black (CB) was covalently attached to a bidentate nitrogen coordination motif with a carbon–carbon bond by spontaneous reaction with an in situ generated ligand precursor. The functionalized support was treated with [Rh(CO)2(μ-Cl)]2 to form a heterogeneous carbon-based support covalently linked to a well defined Rh(I) coordination complex. The hybrid material was characterized using X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), Infrared (IR) spectroscopy and inductively coupled plasma mass spectrometry (ICP-MS). The CB-supported Rh(I) catalyst was active in both hydroamination and dihydroalkoxylation reactions achieving turnover numbers approaching 1000 and was readily recycled. The selectivity of an intramolecular dihydroalkoxylation reaction was significantly improved by covalently anchoring the catalyst to the CB surface.

 Please wait while we load your content...
Please wait while we load your content...