Displacement of ethene from the decamethyltitanocene-ethene complex with internal alkynes, substituent-dependent alkyne-to-allene rearrangement, and the electronic transition relevant to the back-bonding interaction†‡
Abstract
The titanocene-ethene complex [Ti(II)(η2-C2H4)(η5-C5Me5)2] (1) with simple internal alkynes R1C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR2 gives complexes [Ti(II)(η2-R1C
CR2 gives complexes [Ti(II)(η2-R1C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR2)(η5-C5Me5)2] {R1, R2: Ph, Ph (3), Ph, Me (4), Me, SiMe3 (5), Ph, SiMe3 (6), t-Bu, SiMe3 (7), and SiMe3, SiMe3 (8). In contrast, alkynes with R1 = Me and R2 = t-Bu or i-Pr afford allene complexes [Ti(II)(η2-CH2
CR2)(η5-C5Me5)2] {R1, R2: Ph, Ph (3), Ph, Me (4), Me, SiMe3 (5), Ph, SiMe3 (6), t-Bu, SiMe3 (7), and SiMe3, SiMe3 (8). In contrast, alkynes with R1 = Me and R2 = t-Bu or i-Pr afford allene complexes [Ti(II)(η2-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHR2)(η5-C5Me5)2] (11) and (12), whereas for R2 = Et a mixture of alkyne complex (13A) and minor allene (13) is obtained. Crystal structures of 4, 6, 7 and 11 have been determined; the latter structure proved the back-bonding interaction of the allene terminal double bond. Only the synthesis of 8 from 1 was inefficient because the equilibrium constant for the reaction [1] + [Me3SiC
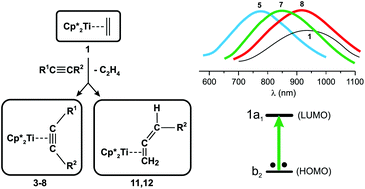
CHR2)(η5-C5Me5)2] (11) and (12), whereas for R2 = Et a mixture of alkyne complex (13A) and minor allene (13) is obtained. Crystal structures of 4, 6, 7 and 11 have been determined; the latter structure proved the back-bonding interaction of the allene terminal double bond. Only the synthesis of 8 from 1 was inefficient because the equilibrium constant for the reaction [1] + [Me3SiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3] ⇌ [8] + [C2H4] approached 1. Compound 9 (R1, R2: Me), not obtainable from 1, together with compounds 3–6 and 10 (R1, R2: Et) were also prepared by alkyne exchange with 8, however this reaction did not take place in attempts to obtain 7. Compounds 1 and 3–9 display the longest-wavelength electronic absorption band in the range 670–940 nm due to the HOMO → LUMO transition. The assignment of the first excitation to be of predominantly a b2 → a1 transition was confirmed by DFT calculations. The calculated first excitation energies for 3–9 followed the order of hypsochromic shifts of the absorption band relative to 8 that were induced by acetylene substituents: Me > Ph ≫ SiMe3. Computational results have also affirmed the back-bonding nature in the alkyne-to-metal coordination.
CSiMe3] ⇌ [8] + [C2H4] approached 1. Compound 9 (R1, R2: Me), not obtainable from 1, together with compounds 3–6 and 10 (R1, R2: Et) were also prepared by alkyne exchange with 8, however this reaction did not take place in attempts to obtain 7. Compounds 1 and 3–9 display the longest-wavelength electronic absorption band in the range 670–940 nm due to the HOMO → LUMO transition. The assignment of the first excitation to be of predominantly a b2 → a1 transition was confirmed by DFT calculations. The calculated first excitation energies for 3–9 followed the order of hypsochromic shifts of the absorption band relative to 8 that were induced by acetylene substituents: Me > Ph ≫ SiMe3. Computational results have also affirmed the back-bonding nature in the alkyne-to-metal coordination.

 Please wait while we load your content...
Please wait while we load your content...