Heterobimetallic metallation studies of N,N-dimethylphenylethylamine (DMPEA): benzylic C–H bond cleavage/dimethylamino capture or intact DMPEA complex†‡
Abstract
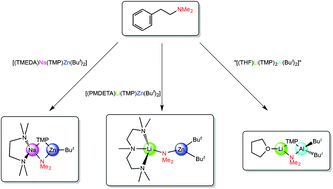
Reaction of the sodium monoamido-bisalkylzincate [(TMEDA)Na(TMP)(But)Zn(But)] (TMEDA is N,N,N′,N′-tetramethylethylenediamine; TMP is 2,2,6,6-tetramethylpiperidide) and the related lithium zincate [(PMDETA)Li(TMP)Zn(But)2] (PMDETA is N,N,N′,N′′,N′′-pentamethyldiethylenetriamine) with the sensitive bio-relevant scaffold N,N-dimethylphenylethylamine, DMPEA, afforded the crystalline complexes [(TMEDA)Na(TMP)(NMe2)Zn(But)] and [(PMDETA)Li(NMe2)Zn(But)2], respectively, both of which have been characterized by NMR spectroscopic and X-ray crystallographic studies. Made by reaction of a LiTMP–(TMP)Al(Bui)2 mixture with DMPEA, a third dimethylamino-containing crystalline complex, the aluminate [(THF)Li(TMP)(NMe2)Al(Bui)2] has been similarly characterized. All three complexes can be regarded as products of cleave and capture chemistry whereby metallation at the benzylic position of DMPEA has led to a β-elimination of an anionic Me2N fragment that has been captured by a charge balancing cationic heterobimetallic entity. While a metallated intermediate prior to the elimination has proved elusive in all of these reactions, DMPEA has been captured fully intact in the novel Lewis acid–Lewis base crystalline complex [DMPEA·Li(TMP)Zn(Me)2], which has also been characterized by the aforementioned techniques.
- This article is part of the themed collection: In memory of Professor Kenneth Wade

 Please wait while we load your content...
Please wait while we load your content...