The effect of steric changes on the isoselectivity of dinuclear indium catalysts for lactide polymerization†
Abstract
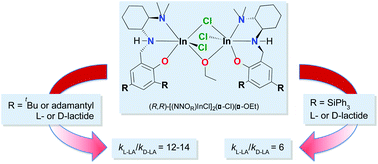
A series of (±)- and (R,R)-tridentate diamino, ortho/para disubstituted phenolate proligands H(NNOR) with various phenolate substituents was synthesized and used to prepare indium dichloride complexes (NNOR)InCl2via salt metathesis of the deprotonated ligands with indium trichloride. These complexes are dinuclear in the solid state, in contrast to previously reported complexes with t-butyl or methyl phenolate substituents. Solution state 1H and PGSE NMR spectroscopy suggests that a fast exchange between the monomeric and dimeric forms of these complexes may exist in solution and is likely influenced by the chirality of the complexes undergoing aggregation. The indium dichloride complexes were utilized to synthesize dinuclear indium ethoxide complexes via salt metathesis with sodium ethoxide. These complexes were active for the polymerization of lactide. In situ and bulk polymerization data confirmed differences in the activity and selectivity of these systems based on the phenolate substituents as well as the ligand chirality.

 Please wait while we load your content...
Please wait while we load your content...