Rapid degradation of cyclic peroxides by titanium and antimony chlorides†
Abstract
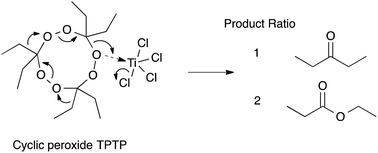
First responders face extraordinary risks when dealing with organic peroxides such as TATP due to the extreme sensitivity of this class of explosives, and to a lack of a robust chemical means of safe and rapid neutralisation. The Lewis acids TiCl4 and SbCl3 have been found to demonstrate a novel degradation mechanism, with TiCl4 degrading a model cyclic peroxide in minutes when used in a two-fold excess, or ∼3 hours at half equivalence. The products cannot re-form peroxide compounds as is the case with acid degradation, suggesting the two mechanisms are fundamentally different. The Lewis acids mediate a rearrangement reaction in the cyclic peroxide backbone leading to relatively innocuous products through deactivation of oxidising O. Sub-stoichiometric TiCl4 reactions highlight a secondary reaction pathway that also leads to some oxidative chlorination products, possibly mediated by an unconfirmed titanium-oxychloride species. SbCl3 was found to exhibit similar reactivity to TiCl4, although at a slower rate. A mechanism is proposed, consistent with the observations for both stoichiometric and sub-stoichiometric quantities of TiCl4.

 Please wait while we load your content...
Please wait while we load your content...