Novel imidazolium and imidazolinium salts containing the 9-nickelafluorenyl anion – synthesis, structures and reactivity†
Abstract
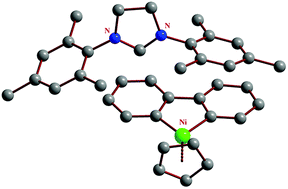
Investigation of the properties of carbene complexes is one of the most important fields of modern coordination chemistry. In this paper, we propose the convenient synthesis of NHC–nickel compounds. The 9-nickelafluorenyllithium complex reacts with imidazolium or imidazolinium salts to afford 9-nickelafluorenyl–NHC salts via ionic metathesis with very good yields (66–92%). These compounds can be isomerised at elevated temperatures to give Ni–NHC complexes with excellent yields (88–91%), probably via nickel mediated hydrogen transfer to the biphenyl moiety. In this reaction, the nickelacyclic ring itself serves as a base in the deprotonation of the carbene precursor. DFT calculations show the thermodynamic instability of the synthesized salts, with Gibbs free energy differences for 1 of −84 kJ mol−1 at 298 K and −167 kJ mol−1 at 374 K. The obtained salts and carbene complexes are relatively air and moisture stable in the solid state.

 Please wait while we load your content...
Please wait while we load your content...