Porous ZrO2 sheets synthesized using an ionothermal method and their absorption properties
Abstract
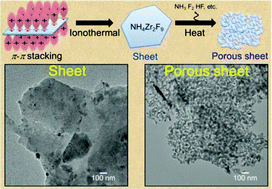
In this study, porous ZrO2 sheets were synthesized using an ionothermal method combined with heat treatments at 400, 600 and 800 °C. Following ionothermal synthesis, NH4Zr2F9 with a sheet-like structure was obtained. After heat treatment, the NH4Zr2F9 was transformed into monoclinic ZrO2 with a porous sheet structure. The ZrO2 was characterized using XRD, FT-IR, FE-SEM TEM, TG-DTA, BET, and zeta potential analysis. The specific surface area of the samples increased with heat treatment temperature, being 12, 17, and 19 m2 g−1 for 400, 600, and 800 °C, respectively. In addition, measurement of the zeta potential of the samples in KCl solution showed that all samples were negatively charged at pH 7, and had different isoelectric points. Adsorption was evaluated using methylene blue and methyl orange and the results indicated that samples heated at different temperatures possessed different selectivities for cationic and anionic dyes.

 Please wait while we load your content...
Please wait while we load your content...