Synthesis and anticancer activity of silver(i)–N-heterocyclic carbene complexes derived from the natural xanthine products caffeine, theophylline and theobromine†
Abstract
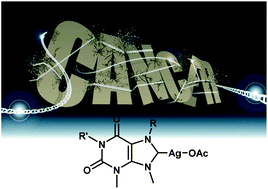
A new library of silver(I)–N-heterocyclic carbene complexes prepared from the natural products caffeine, theophylline and theobromine is reported. The complexes have been fully characterised using a combination of NMR spectroscopy, mass spectrometry, elemental analysis and X-ray diffraction analysis. Furthermore, the hydrophobicity of the complexes has been measured. The silver(I)–N-heterocyclic carbenes have been evaluated for their antiproliferative properties against a range of cancer cell lines of different histological types, and compared to cisplatin. The data shows different profiles of response when compared to cisplatin in the same panel of cells, indicating a different mechanism of action. Furthermore, it appears that the steric effect of the ligand and the hydrophobicity of the complex both play a role in the chemosensitivity of these compounds, with greater steric bulk and greater hydrophilicity delivering higher cytotoxicity.

 Please wait while we load your content...
Please wait while we load your content...