Bidentate NHC^pyrozolate ligands in luminescent platinum(ii) complexes†
Abstract
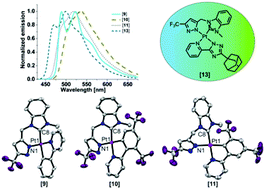
A bidentate C^N donor set derived from an N-heterocyclic carbene (NHC) precursor linked to a trifluoromethyl (CF3) functionalized pyrazole ring is described for the first time. The ligands have been employed to prepare four new phosphorescent complexes by the coordination of platinum(II) centres bearing cyclometalated phenyl-pyridine/triazole-pyridine chelates. The electronic and steric environments of these complexes were tuned through the incorporation of suitable substituents in the phenyl-pyridine/triazole-pyridine ligands, wherein the position of the phenyl-ring substituent (a CF3 group) also directs the selective adoption of either a trans or a cis configuration between the CNHC and the Cphenyl donor atoms. Molecular structures obtained by X-ray diffraction for three of the complexes confirm a distorted square-planar configuration around the platinum centre, and DFT calculations show that the substituents have a significant influence on the energies of the frontier orbitals. Moreover, a platinum(II) complex featuring the new bidentate NHC^pyrazolate ligand and a bulky adamantyl functionalized pyridine-triazole luminophore was observed to be highly emissive and exhibiting a sky-blue luminescence (λEm = 470 nm) with photoluminescence quantum yields as high as 50% in doped PMMA matrices. A complete photophysical investigation of all of the complexes in solution as well as in the solid state is herein reported.
- This article is part of the themed collection: Luminescent Complexes and Materials for Light-Emitting Devices

 Please wait while we load your content...
Please wait while we load your content...