“3 + 1 = 6 + 2” in Cu(ii) coordination chemistry of 1H-pyrazole aza cryptands†
Abstract
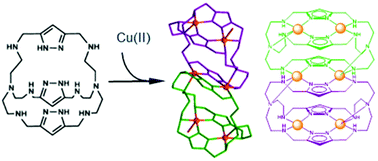
A polyazamacrocycle formed from two tris(2-aminoethyl)amine units connected by 1H-pyrazole units shows unique hexanuclear Cu(II) complexes by combination of two binuclear Cu(II) cryptand complexes through pyrazolate moieties belonging to both cryptands. The formation of these dimeric entities has been proven both in solution by potentiometric studies and mass spectroscopy and in the solid state by X-ray diffraction of crystals of three different batches of formulae [Cu6(H-3L)2(H2O)2](TsO)6·22H2O (2), [Cu6(H-3L)2(NO3)2](NO3)4·2H2O (3) and [Cu6(H-3L)2Cl2]Cl4·(C4H5N3O2)2·14.35H2O (4). The hexanuclear unit in 2 and 4 can be viewed like three magnetically independent binuclear complexes with J = −366(3) cm−1, g = 2.08(1) for 2 and J = −360(3) cm−1, g = 2.07(1) for 4.

 Please wait while we load your content...
Please wait while we load your content...