Unprecedented structural variations in trinuclear mixed valence Co(ii/iii) complexes: theoretical studies, pnicogen bonding interactions and catecholase-like activities†
Abstract
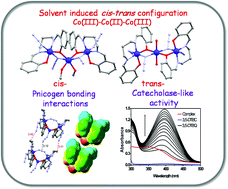
Three new mixed valence trinuclear Co(II/III) compounds cis-[Co3L2(MeOH)2(N3)2(μ1,1-N3)2] (1), trans-[Co3L2(H2O)2(N3)2(μ1,1-N3)2]·(H2O)2 (2) and [Co3LR2(N3)3(μ1,3-N3)] (3) have been synthesized by reacting a di-Schiff base ligand (H2L) or its reduced form [H2LR] (where H2L = N,N′-bis(salicylidene)-1,3-propanediamine and H2LR = N,N′-bis(2-hydroxybenzyl)-1,3-propanediamine) with cobalt perchlorate hexahydrate and sodium azide. All three products have been characterized by IR, UV-Vis and EPR spectroscopies, ESI-MS, elemental, powder and single crystal X-ray diffraction analyses. Complex 1 is an angular trinuclear species in which two terminal octahedral Co(III)N2O4 centers coordinate to the central octahedral cobalt(II) ion through μ2-phenoxido oxygen and μ1,1-azido nitrogen atoms along with two mutually cis-oxygen atoms of methanol molecules. On the other hand, in linear trinuclear complex 2, in addition to the μ2-phenoxido and μ1,1-azido bridges with terminal octahedral Co(III) centres, the central Co(II) is bonded with two mutually trans-oxygen atoms of water molecules. Thus the cis–trans configuration of the central Co(II) is solvent dependent. In complex 3, the two terminal octahedral Co(III)N2O4 centers coordinate to the central penta-coordinated Co(II) ion through double phenoxido bridges along with the nitrogen atom of a terminal azido ligand. In addition, the two terminal Co(III) are connected through a μ1,3-azido bridge that participates in pnicogen bonding interactions (intermolecular N–N interaction) as an acceptor. Both the cis and trans isomeric forms of 1 and 2 have been optimized using density functional theory (DFT) calculations and it is found that the cis configuration is energetically more favorable than the trans one. However, the trans configuration of 2 is stabilized by the hydrogen bonding network involving a water dimer. The pnicogen bonding interactions have been demonstrated using MEP surfaces and CSD search which support the counter intuitive electron acceptor ability of the μ1,3-azido ligand. Complexes 1–3 exhibit catecholase-like activities in the aerial oxidation of 3,5-di-tert-butylcatechol to the corresponding o-quinone. Kinetic data analyses of this oxidation reaction in acetonitrile reveal that the catecholase-like activity follows the order: 1 (kcat = 142 h−1) > 3 (kcat = 99 h−1) > 2 (kcat = 85 h−1). Mechanistic investigations of the catalytic behaviors by X-band EPR spectroscopy and estimation of hydrogen peroxide formation indicate that the oxidation reaction proceeds through the reduction of Co(III) to Co(II).


 Please wait while we load your content...
Please wait while we load your content...