One-pot synthesis of an indole-substituted 7,8-dicarba-nido-dodecahydroundecaborate(−1)†
Abstract
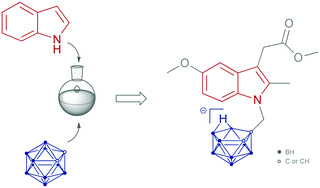
Carbaboranes are increasingly used as pharmacophores to replace phenyl substituents in established drug molecules. In contrast to traditional organic chemistry, elaborate procedures to introduce functionality frequently fail in the case of carbaboranes and their chemistry is often hampered by degradation of the cluster. Herein, the development of a one-pot synthesis of a water-soluble N-nido-dicarbaborato indole is presented, including a proposed mechanism for the reaction sequence. These studies provide useful synthetic tools for the conjugation of two important pharmacophores, indoles and carbaboranes.

 Please wait while we load your content...
Please wait while we load your content...