Synthesis and characterization of μ-nitrido, μ-carbido and μ-oxo dimers of iron octapropylporphyrazine†
Abstract
Three μ-X bridged diiron octapropylporphyrazine complexes having FeIII–O–FeIII, Fe+3.5–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Fe+3.5 and FeIV
Fe+3.5 and FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) FeIV structural units have been prepared and characterized by UV-vis, EPR, X-ray absorption spectroscopy and electrochemical methods. Single crystals of all the complexes were obtained from benzene-acetonitrile and their structures were determined by X-ray diffraction. In contrast to μ-oxo complex (6), μ-nitrido (7) and μ-carbido (8) dimers crystallized with one benzene molecule per two binuclear complex molecules arranged cofacially to the porphyrazine planes at Fe–Cbenzene distances of 3.435–3.725 Å and 3.352–3.669 Å for 7 and 8, respectively. The short distances suggest an interaction between the iron sites and the benzene π-system which is stronger in the case of the FeIV
FeIV structural units have been prepared and characterized by UV-vis, EPR, X-ray absorption spectroscopy and electrochemical methods. Single crystals of all the complexes were obtained from benzene-acetonitrile and their structures were determined by X-ray diffraction. In contrast to μ-oxo complex (6), μ-nitrido (7) and μ-carbido (8) dimers crystallized with one benzene molecule per two binuclear complex molecules arranged cofacially to the porphyrazine planes at Fe–Cbenzene distances of 3.435–3.725 Å and 3.352–3.669 Å for 7 and 8, respectively. The short distances suggest an interaction between the iron sites and the benzene π-system which is stronger in the case of the FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
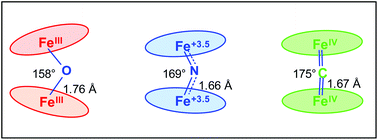
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) FeIV unit with a higher Lewis acidity. The Fe–X–Fe angle increases in the sequence 6–7–8 from 158.52° to 168.5° and 175.10°, respectively, in agreement with the Fe–X bond order. However, the lengths of the Fe–X bonds do not follow this trend: Fe–O = 1.75/1.76 Å > Fe–C = 1.67/1.67 Å > Fe–N = 1.65/1.66 Å indicating unexpectedly long Fe–C bonds. This observation can be explained by back π-donation from the μ-carbido ligand to the Fe–C antibonding orbital thus decreasing the bond order which is confirmed by DFT calculations.
FeIV unit with a higher Lewis acidity. The Fe–X–Fe angle increases in the sequence 6–7–8 from 158.52° to 168.5° and 175.10°, respectively, in agreement with the Fe–X bond order. However, the lengths of the Fe–X bonds do not follow this trend: Fe–O = 1.75/1.76 Å > Fe–C = 1.67/1.67 Å > Fe–N = 1.65/1.66 Å indicating unexpectedly long Fe–C bonds. This observation can be explained by back π-donation from the μ-carbido ligand to the Fe–C antibonding orbital thus decreasing the bond order which is confirmed by DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...