π-Excess aromatic σ2-P ligands: synthesis and structure of an unprecedented μ2-P-1,3-benzazaphosphole bridged tetranuclear copper(i) acetate complex†‡
Abstract
The –P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–NR-heterocycle 1-neopentyl-1,3-benzazaphosphole (npBAP) reacts in THF with copper(I) acetate via labile soluble complexes (npBAP)xCuOAc preferably to form the insoluble tetranuclear (npBAP)2(CuOAc)4 complex 1. The crystal structure analysis of 1·2CH2Cl2, grown from CH2Cl2–hexane, reveals two μ2-P coordinated ligands, folding the Cu4 ring with each two short distances of the cyclic copper(I) acetate tetramer to a butterfly arrangement. AIM analysis of the ωB97-D/6-31+G* electron density, obtained at the crystal structure geometry, shows bond critical points between the copper atoms indicating covalent bonding interactions between the neighboring Cu atoms.
CH–NR-heterocycle 1-neopentyl-1,3-benzazaphosphole (npBAP) reacts in THF with copper(I) acetate via labile soluble complexes (npBAP)xCuOAc preferably to form the insoluble tetranuclear (npBAP)2(CuOAc)4 complex 1. The crystal structure analysis of 1·2CH2Cl2, grown from CH2Cl2–hexane, reveals two μ2-P coordinated ligands, folding the Cu4 ring with each two short distances of the cyclic copper(I) acetate tetramer to a butterfly arrangement. AIM analysis of the ωB97-D/6-31+G* electron density, obtained at the crystal structure geometry, shows bond critical points between the copper atoms indicating covalent bonding interactions between the neighboring Cu atoms.
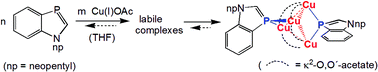

 Please wait while we load your content...
Please wait while we load your content...