Organic–inorganic hybrid rare earth complexes based on polymolybdates with intrinsic photosensitive properties†
Abstract
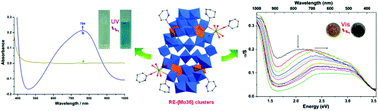
A series of organic–inorganic hybrid rare earth complexes {[RE2(PO)2(H2O)10][H2Mo36O112(OH2)12(PO)4]}·5PO·2(CH3CN)·nH2O [n = 23–42, RE(III) = Nd(III), 1; Sm(III), 2; Eu(III), 3; Gd(III), 4; Dy(III), 5; Er(III), 6; Tm(III), 7; Yb(III), 8; Lu(III), 9; Y(III), 10; PO = piperidin-2-one] have been synthesized and fully characterized by single-crystal X-ray diffraction, powder X-ray diffraction, elemental analysis, IR spectra, thermogravimetric analysis and UV-vis spectra. Structural analysis reveals that compounds 1–10 are isostructural and crystallize in the monoclinic P2(1)/n space group. Each compound contains a centrosymmetric anionic cluster [Mo36O112(OH2)12(PO)4]8−, which could be described as the derivative of [Mo36O112(OH2)16]8− with four water molecules substituted by organic PO molecules. Each {Mo18} subunit connects with one RE(III) ion via its two terminal O atoms from two independent {MoO6} octahedra. The eight coordinated RE(III) ion with a distorted tetragonal antiprism coordination geometry is also surrounded by another six oxygen atoms, five of them from five water molecules and the final one from one PO molecule. Compounds 1–10 show considerable photosensitive behavior under visible light excitation. In addition, compound 3 exhibits three emission bands at 580, 595 and 617 nm in the solid state, which could be assigned to 5D0→7F0, 5D0→7F1 and 5D0→7F2 transitions of Eu(III) ions, respectively.

 Please wait while we load your content...
Please wait while we load your content...