PET/PDT theranostics: synthesis and biological evaluation of a peptide-targeted gallium porphyrin†
Abstract
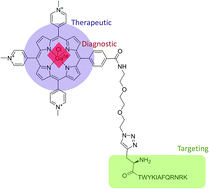
The development of novel theranostic agents is an important step in the pathway towards personalised medicine, with the combination of diagnostic and therapeutic modalities into a single treatment agent naturally lending itself to the optimisation and personalisation of treatment. In pursuit of the goal of a molecular theranostic suitable for use as a PET radiotracer and a photosensitiser for PDT, a novel radiolabelled peptide–porphyrin conjugate targeting the α6β1-integrin has been developed. 69/71Ga and 68Ga labelling of an azide-functionalised porphyrin has been carried out in excellent yields, with subsequent bioconjugation to an alkyne-functionalised peptide demonstrated. α6β1-integrin expression of two cell lines has been evaluated by flow cytometry, and therapeutic potential of the conjugate demonstrated. Evaluation of the phototoxicity of the porphyrin–peptide theranostic conjugate in comparison to an untargeted control porphyrin in vitro, demonstrated significantly enhanced activity for a cell line with higher α6β1-integrin expression when compared with a cell line exhibiting lower α6β1-integrin expression.
- This article is part of the themed collection: Dalton Discussion 15: Metal ions in medical imaging

 Please wait while we load your content...
Please wait while we load your content...