Mono-, di- and tetra-zinc complexes derived from an amino-benzotriazole phenolate ligand containing a bulkier N-alkyl pendant arm: synthesis, structure and catalysis for ring-opening polymerization of cyclic esters†
Abstract
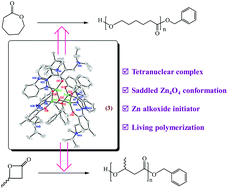
Zinc complexes constructed from the amino-modified benzotriazole phenol pro-ligand, 2-(2H-benzotriazol-2-yl)-6-((diisopropylamino)methyl)-4-(2,4,4-trimethylpentan-2-yl)phenol (C8DIABTP-H, 1), were synthesized stepwise and structurally characterized. The reaction of C8DIABTP-H (1) with one equivalent of diethyl zinc (ZnEt2) generates a dimeric and four-coordinated zinc complex, [(μ-C8DIABTP)ZnEt]2 (2), which is doubly bridged by two phenolate groups of C8DIABTP ligands. Further reaction of 2 with benzyl alcohol (BnOH) in stoichiometric proportions affords a tetranuclear zinc benzylalkoxide complex [(μ-OBn)(C8DIABTP)Zn]4 (3) that possesses a saddle-shaped core with four μ2-bridging benzylalkoxy groups upon four Zn centres. Interestingly, the di-nuclear Zn alkoxide [(μ-OBn)(C8DIABTP)Zn(DMAP)]2 (4) could be prepared by treatment of 3 with a stoichiometric amount of 4-(dimethylamino)pyridine (DMAP). ZnEt2 reacts with two equivalents of 1 in the presence of DMAP (1.0 mol equiv.) to yield a five-coordinated mononuclear zinc complex, [(C8DIABTP)2Zn(DMAP)] (5). All complexes adopt an N,O-bidentate coordination mode from the phenoxy oxygen atom and benzotriazole nitrogen atom, in which the nitrogen atom of the pendent arm substituent is not coordinated to the zinc centre. Ring-opening polymerization of ε-caprolactone and β-butyrolactone catalysed by 2 and 3 was investigated.
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...