Assembly of three coordination polymers based on a sulfonic–carboxylic ligand showing high proton conductivity†
Abstract
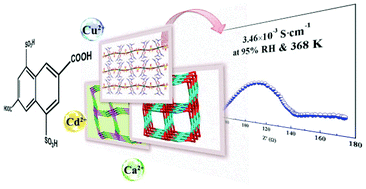
Three new coordination polymers (CPs)/metal–organic frameworks (MOFs) with different structures have been synthesized using 4,8-disulfonyl-2,6-naphthalenedicarboxylic acid (H4L) and metal ions, Cu2+, Ca2+ and Cd2+. The Cu compound features a one-dimensional chain structure, further extending into a 2D layer network through H-bond interactions. Both the Ca and Cd compounds show 3D frameworks with (4,4)-connected PtS-type topology and (3,6)-connected bct-type topology, respectively. These CPs/MOFs all exhibit proton conduction behavior, especially for the Cu compound with a proton conductivity of 3.46 × 10−3 S cm−1 at 368 K and 95% relative humidity (RH). Additionally, the activation energy (Ea) has also been investigated to deeply understand the proton-conduction mechanism.

 Please wait while we load your content...
Please wait while we load your content...